FDA Approves Stroke Rehabilitation Therapy Created at UT Dallas
By: Stephen Fontenot | Sept. 10, 2021

For the 7 million American survivors of stroke, increasing the effectiveness of physical rehabilitation for mobility and motor skills could provide a transformative boost in quality of life.
Researchers from The University of Texas at Dallas’ Texas Biomedical Device Center (TxBDC) conceived a therapy to rewire circuits in the brain involving vagus nerve stimulation (VNS) more than a decade ago, and scientists have since been refining the technique to treat a variety of disorders, including stroke. On Aug. 27, the Food and Drug Administration (FDA) approved the rehabilitation system for chronic ischemic stroke survivors — the first such treatment of its kind.
The Vivistim Paired VNS System is produced and commercialized by MicroTransponder, a spinoff company started by UT Dallas graduates. The results of a pivotal double-blind, placebo-controlled, randomized clinical trial — published April 24 in The Lancet — showed that in patients with arm and hand weakness after stroke, pairing VNS with rehabilitation exercises led to improvements that were two to three times greater than the control group receiving rehabilitation alone.
Dr. Michael Kilgard, the Margaret Fonde Jonsson Professor of neuroscience in the School of Behavioral and Brain Sciences (BBS) and interim executive director of the TxBDC, described FDA approval of the system as “the most significant success” in the center’s nine-year history.
“This announcement means that stroke survivors all over the country can soon begin receiving a safe and effective therapy to improve their recovery,” Kilgard said.
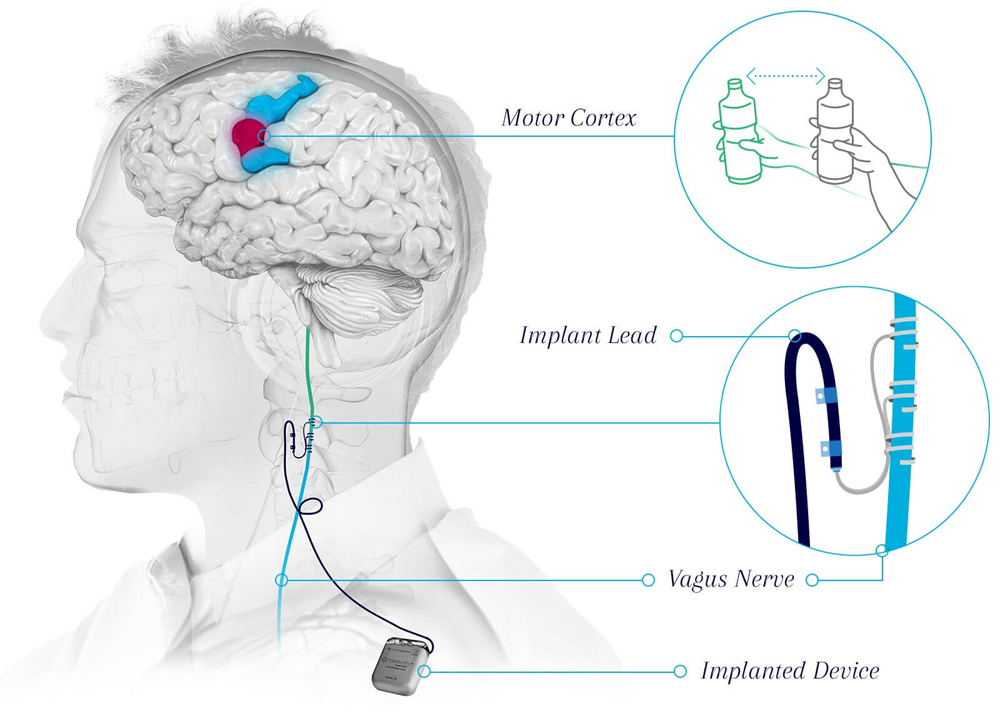
The FDA also gave Vivistim a Breakthrough Device Designation, which is reserved for technology that treats life-threatening or irreversibly debilitating conditions and has significant advantages over any existing alternatives.
The vagus nerve travels up the neck from the chest and abdomen and relays information between the brain and the body, regulating processes like digestion, heart rate and respiratory rate. In VNS, an implanted device stimulates the nerve with electrical impulses. Similar devices are currently FDA-approved to treat epilepsy and depression.
Researchers affiliated with UT Dallas’ TxBDC, BBS and the Erik Jonsson School of Engineering and Computer Science developed the technique of targeted plasticity therapy, now called paired VNS, which pairs movements with electrical stimulation delivered to the vagus nerve via an implanted device in the neck.
“The first studies of our technique in humans showed that by adding VNS, patients can recover to a degree that they would not otherwise — even if they start 10 years after their stroke.”
Dr. Michael Kilgard, the Margaret Fonde Jonsson Professor of neuroscience in the School of Behavioral and Brain Sciences and interim executive director of the Texas Biomedical Device Center
“The idea is that we can’t fix the region of the brain that’s been damaged by stroke, but we can help the remainder of the brain rewire to get the job done,” Kilgard said. “This recovery doesn’t happen spontaneously.”
Kilgard explained that, for the two-thirds of stroke survivors who are chronically disabled, the potential for recovery via physical therapy after a stroke is usually limited to the first six months.
“In that initial period after a stroke, people can regain some use of their upper limbs, but usually there’s not much recovery after that. Most people plateau even if they keep working at it,” he said. “But the first studies of our technique in humans showed that by adding VNS, patients can recover to a degree that they would not otherwise — even if they start 10 years after their stroke.”
The initial work with VNS at UT Dallas began with Kilgard and Dr. Robert Rennaker, founding director and chief technology officer for the TxBDC and the Texas Instruments Distinguished Chair in Bioengineering. Their research was initially aimed at treating tinnitus — the perception of sound, like a ringing in the ears, with no external source.
VNS Clinical Trials
Texas Biomedical Device Center researchers continue to conduct clinical trials using VNS to treat stroke, spinal cord injury and post-traumatic stress disorder. For information about becoming a participant, visit the TxBDC clinical trials website.
To learn more about how the University is enhancing lives through transformative research, visit the New Dimensions: The Campaign for UT Dallas website.
“This long road began with a paper in Nature in 2011 about treating tinnitus,” Kilgard said. “The founders of MicroTransponder stayed in Dallas to commercialize paired VNS therapy for both stroke and tinnitus.”
Navzer Engineer PhD’04, chief scientific officer at MicroTransponder, worked with Kilgard on the initial preclinical VNS studies, which were supported at the time with a $1.7 million grant from the National Institutes of Health.
“Our stroke study published in The Lancet was the culmination of a large amount of preclinical research that we started over a decade ago at UT Dallas,” Engineer said. “This would not have been possible without a dedicated team of amazing students and researchers at UTD.”
Dr. Seth Hays, associate professor of bioengineering in the Jonsson School, started working on the project as a postdoctoral student in spring 2012, joining then-graduate student Navid Khodaparast BS’06, MS’09, PhD’13 and Ben Porter MS’11, PhD’11.
Hays described targeted plasticity therapy and VNS for stroke as “the definition of grassroots” — the approach was invented and developed at UT Dallas, by UT Dallas students, staff and faculty.
“We’ve taken the opportunity to develop this here, and a lot of people in Texas should be proud of it — the individuals who enrolled in the study, the team at MicroTransponder, the medical centers that were involved and the University,” Hays said.
“From its inception, the Texas Biomedical Device Center has worked as a team with a singular focus of providing patients with new hope for greater recovery. All of the preclinical and much of the clinical research that led VNS for stroke from an invention to an approved therapy was conducted right here.”
Dr. Robert Rennaker, founding director and chief technology officer for the Texas Biomedical Device Center and the Texas Instruments Distinguished Chair in Bioengineering
Kilgard said 22 TxBDC-affiliated researchers have been authors of the papers that reported early preclinical and clinical results for the stroke therapy, supported in the scientific process by more than 200 TxBDC students and staff.
“From its inception, the Texas Biomedical Device Center has worked as a team with a singular focus of providing patients with new hope for greater recovery,” Rennaker said. “All of the preclinical and much of the clinical research that led VNS for stroke from an invention to an approved therapy was conducted right here.”
The TxBDC at UT Dallas was established in 2012 with donations from Texas Instruments Inc., a private donor and matching funds from UT System Regents’ Research Incentive Program. Since then, it has generated more than $35 million in additional funding to develop technologies to prevent injuries, detect impairments and restore quality of life lost due to neurological injuries and disease.
Media Contact:
Stephen Fontenot, UT Dallas, 972-883-4405, stephen.fontenot@utdallas.edu, or the Office of Media Relations, UT Dallas, (972) 883-2155, newscenter@utdallas.edu.





