Team Engineers New Way To Get Medication Past Blood-Brain Barrier
By: Kim Horner | Nov. 10, 2021

A University of Texas at Dallas-led team of researchers has developed a new technique to open the blood-brain barrier temporarily to deliver medication to the brain.
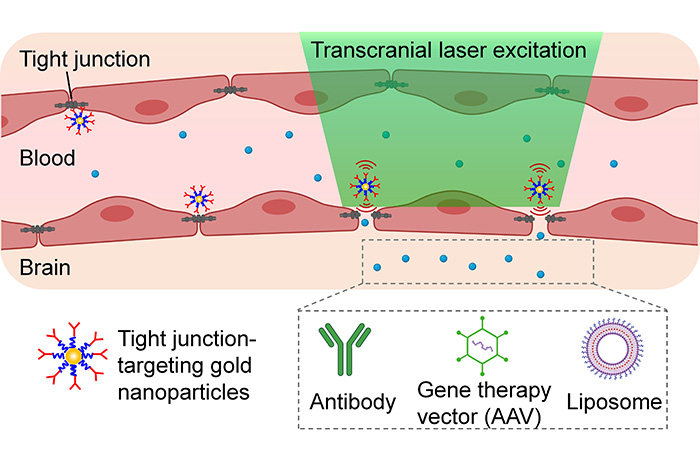
Getting medication past the brain’s unique and protective blood vessels, known as the blood-brain barrier, is one of the biggest challenges in treating brain and central nervous system diseases, said Dr. Zhenpeng Qin, associate professor of mechanical engineering at UT Dallas and co-corresponding author of the study that describes the method. The technique uses light and nanoparticles to pry open temporarily these barriers — called tight junctions — to allow medication to reach its target.
Qin and his colleagues in the Erik Jonsson School of Engineering and Computer Science and at other institutions demonstrated the approach in mice in a study published online Sept. 13 in the journal Nano Letters.
The findings are the result of five years of research funded in part by the Cancer Prevention and Research Institute of Texas (CPRIT). Qin said the approach could lead to treatments for brain tumors and Lou Gehrig’s disease, also known as amyotrophic lateral sclerosis; aid in stroke recovery; and deliver gene therapy. Qin said further development and testing is needed before it could be used in humans.
“Approaches to increase blood-brain barrier [BBB] permeability are essential to advance therapeutics for central nervous system diseases,” said Xiaoqing Li, the paper’s co-lead author and a biomedical engineering doctoral student at UT Dallas.
The technique involves injecting gold nanoparticles, which absorb light, into the bloodstream to target the blood-brain barrier. Researchers apply picosecond (one-trillionth of a second) laser pulses externally to activate the gold nanoparticles.

To learn more about how UT Dallas is enhancing lives through transformative research, explore New Dimensions: The Campaign for UT Dallas.
“The action produces a tiny mechanical force that temporarily breaks the barrier open so a drug can enter the blood flow into the brain,” Li said.
The study demonstrated that the technique did not damage the blood-brain barrier or the constriction and dilation of blood vessels, called vasomotion.
“We demonstrated that the BBB permeability can be modulated without significant disruption to the spontaneous vasomotion or the structure of the neurovascular unit,” said Dr. Qi Cai, mechanical engineering research associate and co-lead author of the paper.
In their experiments, the researchers tested the method with cargos of antibodies, liposomes and adeno-associated viral vectors, which can be used to carry gene-editing components.
In August, Qin received a fourth CPRIT grant to study whether the method can be used to treat glioblastoma, the most common malignant brain tumor in adults. He and his team aim to design and produce magnetic nanoparticles that can be stimulated to disrupt the blood-brain barrier using magnetic fields.
“Support from CPRIT has been instrumental in our work,” said Qin, who received grants from the state agency — the second-largest cancer research and prevention program in the world — in 2016, 2018 and 2019. “When we started, we had an idea, basically to use nanoparticles to target specific components of the blood-brain barrier with minimal injury.”

The Nano Letters study involved a global interdisciplinary team of researchers including Dr. Heather Hayenga and Dr. Shashank Sirsi, both assistant professors of bioengineering at UT Dallas. Additional UT Dallas co-authors included Dr. Hejian Xiong, research scientist in mechanical engineering; Peiyuan Kang PhD’20, now a postdoctoral researcher at Harvard Medical School; and Dr. Xiuying Li, now a scientist at Nexus Pharmaceuticals Inc.
Other collaborators included researchers at UT Southwestern Medical Center: co-corresponding author Dr. Robert Bachoo, associate professor of neurology and internal medicine; Dr. Vamsidhara Vemireddy, co-lead author; and Dr. Edward Pan, associate professor of neurology and neurological surgery and section head of neuro-oncology.
The collaboration also included researchers from the Italian Foundation for Cancer Research Institute of Molecular Oncology, the Smurfit Institute of Genetics at Trinity College Dublin, and the University of California, San Diego.
In addition to CPRIT, funding for the research came from the American Heart Association, the National Institutes of Health, the European Research Council and Fondazione Cariplo.
Grant Aids Work on Tool To Study Neuropeptides

Dr. Zhenpeng Qin, associate professor of mechanical engineering at The University of Texas at Dallas, has received a $1 million National Science Foundation award to develop nanotechnology to help scientists study how chemical messengers called neuropeptides affect brain activity.
The four-year project is part of a $3 million interdisciplinary project with collaborators at Icahn School of Medicine at Mount Sinai and Massachusetts General Hospital, a teaching hospital of Harvard Medical School.
Qin, lead principal investigator on the project, is developing a new technique to make it possible for scientists to control the release of neuropeptides in the brain in order to track the molecules, study their action on brain circuits and examine how they affect behavior.
“Ultimately, we want to use the information to better diagnose and treat brain diseases,” Qin said. “Before we can get there, we need to develop tools to modulate how neuropeptides work in the brain.”
Neuropeptides are a type of neurotransmitter that play a role in regulating cognition and how the central nervous system responds to sensory messages. The way in which they work in the brain, however, is not well understood because of a lack of tools to study them.
While some types of neurotransmitters send messages directly to other cells, neuropeptides broadcast information widely, Qin said.
“The way neuropeptides transmit messages is more like a Wi-Fi signal,” Qin said. “That makes it challenging to study how the molecules affect brain activity.”

The new project builds on Qin’s previous work to deliver medication to targeted areas in the brain using microscopic, molecule-carrying capsules called nanovesicles coated with gold, which makes them sensitive to light.
While that project used lasers to cause the nanovesicles to open and release their cargo, the new project will use light pulses to activate a photoswitch in the nanovesicles to release neuropeptides.
The Erik Jonsson School of Engineering and Computer Science researcher is working with co-principal investigator Dr. Steven Nielsen, associate professor of chemistry in the School of Natural Sciences and Mathematics, who will develop computer models to help study the process.
“We’ll be asking questions, such as ‘How does the nanovesicle change its structure to let its cargo out?’ and ‘What are the implications of changing the nanovesicle’s shape?’” Nielsen said.
Researchers at the Icahn School of Medicine at Mount Sinai will develop cell-based neurotransmitter fluorescent-engineered reporters (CNiFERS) to help researchers image changes in brain neuropeptide levels. Combined with nanovesicles, researchers at Mount Sinai will study the impact of neuropeptide signaling on brain function.
“Ultimately, we want to use the information to better diagnose and treat brain diseases. Before we can get there, we need to develop tools to modulate how neuropeptides work in the brain.”
Dr. Zhenpeng Qin, associate professor of mechanical engineering in the Erik Jonsson School of Engineering and Computer Science
“The exciting thing about this project is that we will be developing the tools that help scientists study how these neuropeptides influence the activity of brain circuits in real time,” said Dr. Paul Slesinger, director of the Center for Neurotechnology and Behavior at Mount Sinai and principal investigator of the project at Mount Sinai.
Dr. Xin Yu, director of the Small Animal MRI Scanning Facility and assistant professor of radiology at Harvard Medical School, will implement a novel multimodal functional MRI platform with fiber photometry, an imaging method that uses signals from fluorescent indicators to monitor neuropeptides’ impact on brainwide activation patterns.
“Our multimodal fMRI platform provides an ideal experimental setup to investigate the impact of neuropeptides on cross-scale brain function from the cellular, neural circuit and systems levels,” Yu said.
Media Contact: Kim Horner, UT Dallas, 972-883-4463, kim.horner@utdallas.edu, or the Office of Media Relations, UT Dallas, (972) 883-2155, newscenter@utdallas.edu.





